Novel cathode material for lithium-sulfur batteries and lithium-ion batteries
Abstract
With the new type of cathode material, the structural and material separation of current collector and cathode material is no longer necessary. In the single-stage strip electroplating process, which dispenses with the addition of binder and electrically conductive filler particles, a quantitatively significantly higher proportion of active material is made possible in the cathode. Li-ion or Li-sulfur cells can thus be operated more efficiently.
Advantages
- Improved properties of battery: Capacity, energy density, efficiency, cycle stability
- Single step manufacturing process
- Reduced cost due to the elimination of binder and conductive particles
- Can be used in Li-Ion cells and Li-Sulfur cells
- Minimal adjustments required to conventional continuous electroplating / electroforming processes
Application
Production of lithium-sulfur batteries and lithium-ion batteries
Background
The demand for high performance batteries is substantial across a diverse range of sectors of the economy. They are required, for example, for mobile IT applications, aerospace, in the area of electric mobility and for battery storage power plants.
The use of the novel cathode material which is the object of the present invention not only increases capacity, energy storage density, energy efficiency and cycle stability, but also reduces the manufacturing costs of lithium-sulfur as well as lithium-ion battery cells.
Problem
In circumstances where the electrochemically active material of a cell has an electrical conductivity which is too low, the cathode material usually consists of a multi-component mixture. For example, carbon particles are added to increase conductivity and binders are added to stabilize the cathode material. The mixture is then applied to the current collector, whose task it is to conduct the electrons to the external circuit.
The inclusion of binding material and electrically conductive particles is not only expensive but also limits the energy density of the cell, given that the volume of binder and conductive particles reduces the volume available for the active material.
A further problem is the relatively high electrical resistance between the surfaces of the conductive particles in the composite cathode material. The cathode mixture has a low overall conductivity and a limited mechanical stability. Furthermore, the integrity of the composite cathode material becomes lower as the cycle number increases, and the capacity as well as the energy efficiency of the cell diminish.
Solution
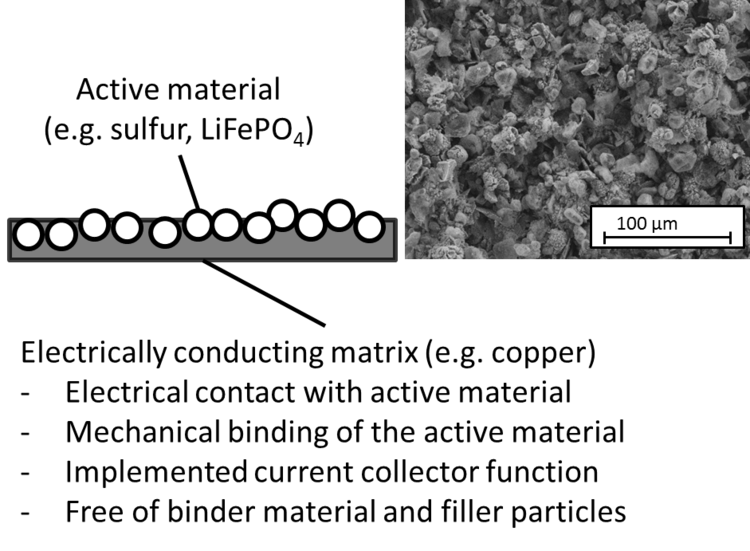
At Aalen University, a novel composite material and a production process was developed which makes the structural and material separation of current collector and cathode material unnecessary. The cathode material can be manufactured and formed in a continuous single-stage electroplating process.
During the manufacture, electrochemically active material (e.g. sulfur or lithium iron phosphate) is homogeneously incorporated in an electrically conductive matrix (e.g. copper). The matrix is used both for conducting the electrons to the external circuit (current collector function) as well as for the direct electrical and mechanical connection with the active material (replacing conducting and binding additives).
The proportion of active material in the cathode can thus be increased, since the addition of binders and electrically conductive particles is no longer required.